Francium, a chemical element with a symbol Fr, is one of the highly reactive alkali metals of group 1 with an atomic number 87 in the periodic table. Francium is not found in a free state in nature as it is highly radioactive i.e. it has a half-life of only 22 minutes. It is the second most rarest naturally occuring element after astatine
As we know how much francium is being used in the world of chemistry, so we must have very good proper information about its electronic properties to survive in the world of chemistry and that’s why you are here to know what valence electrons and valency of francium are, aren’t you? But for this you have to know what these two terms are, so without wasting your time let's go for it,
Difference between valence electrons and valency
Valence electrons are the total number of electrons present in the outermost shell of an atom (i.e. in outermost orbital). The valence electrons for a neutral atom is always definite, it cannot be varied (more or less) in any condition for a particular atom and may or not be equal to its valency.
Valency is defined as the total number of electrons an atom can lose, gain, or share at the time of bond formation to get a stable electronic configuration i.e. to complete an octet. The valency of an atom can be variable in different compounds or in chemical reactions due to different bonding mechanisms.Francium (Fr) valence electrons
There are four simple steps to find out the valence electrons for francium atom which are:
Step 1: Find the Atomic Number
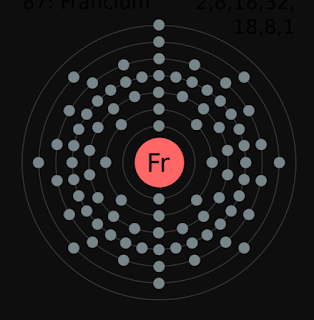
To find out the atomic number of francium, we can use the periodic table. With the help of the periodic table, we can easily see that the atomic number of francium is 87. As its atomic number is 87, it has a total of 87 protons, and for neutral francium, the number of protons is always equal to the number of electrons i.e. 87 electrons in the nucleus.
Step 2: Write Electron Configuration
Electron configuration is the arrangement of electrons on the orbitals. The francium atom has a total of 87 electrons, so we have to put 87 electrons in orbitals. The first two electrons will go in the 1s orbital as S orbital can hold a maximum of two electrons only. The next two will go in 2s orbital and the next six electrons will go in 2p orbital as P orbital can only hold a maximum of 6 electrons and so on...
Step 3: Determine Valence Shell
As we know, the valence shell of an atom can be found from the highest number of principle quantum numbers which is expressed in the term of n, and in [Rn]7s1, the highest value of n is 7 so that the valence shell of Fr is 7s1.
Step 4: Find Valence Electrons
The total number of electrons present in the valence shell of an atom is called valence electrons, and there is only one electron present in the valence shell of francium (7s1). Thus, francium has only one valence electron.
Valency of Francium (Fr)
There are many different ways to find out the valency of an
atom which reflects the ability of an atom to bond with other atoms. Valence describes
how easily an atom or a free radical can combine with other chemical species. The
valency of an atom is determined based on the number of electrons lost, gained, or shared with another atom.
An atom is said to be stable when its outermost shells have
eight electrons (except H and He). If the total number of electrons in
outermost shells is between one to four, the atom has positive valency and if
electrons are between four to eight, the valency is calculated by subtracting
from eight and valency is negative. Atoms having four outermost electrons
possess both positive and negative valency and atoms having eight outermost
electrons have zero valencies (i.e. noble gases).
Alkali metals like francium reached the stable (nearest inert
gas configuration) by losing one outermost electron. So that the valency of francium (Fr) is 1.
We can also find the valency of francium with the help of a periodic table. as francium is an element of group 1 which indicated alkali
metals group and valency of alkali metals are always 1.
Valence electrons and valency of Fr+
Francium-ion Fr+ means it has lost one electron and has only 86 electrons in the orbitals. The electron configuration of neutral Fr is [Rn]7s1 but in Fr+ it loses one electron, so it has a new electron configuration of 1s22s22p63s23p6
Fr+ valency is not zero like noble gas as their outermost
shell has eight electrons. when a francium atom loses one electron, an Fr+ ion is produced and that’s what valency is. So that Fr+ valency is +1, not zero.
Chemical Properties
Francium (Fr) atom| Atomic number | 87 |
| Number of protons | 87 |
| Number of electrons | 87 |
| Electron configuration | [Rn]7s1 |
| Valence electrons | 1 |
| Valence/Valency | 1 |
| Number of electrons | 86 |
| Electronic configuration | 1s22s22p63s23p6 |
| Valence electrons | 8 |
| Valence/Valency | +1 |

Post a Comment