Arsenic pentafluoride is the chemical name of AsF5 which is a toxic and colorless gas. The oxidation state of Arsenic (As) in the molecule is +5. Its molar mass is 169.9136 g/mol, density is 2.138kg/m3, melting point is -79.8 °C, and the boiling point is -52.8 °C.
There are
mainly two methods for the preparation of arsenic pentafluoride, one is a direct combination
of arsenic and fluoride and another is with the reaction between arsenic
trifluoride and fluorine gas.
1. 2 As + 5 → F2 2 AsF5
2. AsF3 + F2 → AsF5
Here in
this article, we are going to learn whether it is a polar molecule or a nonpolar
molecule in detail. But before that first have some ideas about what polar and
nonpolar molecules are:
Polar Molecule
Those molecules
that have a net dipole charge are called polar molecules. In simple terms, if
there is an electronegativity difference between two atoms within the molecule,
induced partial positive and negative charges on either end of the bond. These
types of bonds are called polar bonds.
But being a polar bond is only not enough to make a molecule polar for that molecule must have
an asymmetrical structure so that the induced charges do not cancel.
Ammonia
(NH3), Water (H2O), etc. are some of the examples of polar molecules.
Nonpolar Molecule
Those
molecules that have zero dipole moment/charge are called nonpolar molecules. It means
if the molecules contain two atoms with two different electronegativity values,
they are referred to as polar molecules. However, a molecule can be nonpolar despite having a polar bond only in the case of the molecule is symmetrical.
Xenon tetrafluoride (XeF4), Carbon dioxide (CO2), etc. are some of the examples of
polar molecules
Checkout:
Difference Between Polar & Nonpolar Molecules With Examples [In Detailed].
So, Is AsF5
polar or nonpolar? AsF5 is a nonpolar molecule despite it has five As-F
polar bonds. This is because AsF5 has a trigonal bipyramidal molecular geometry
which a symmetrical structure. The induced charges due to As-F bonds get cancel
and the molecule has a zero net charge.
AsF5 Polar or Nonpolar (Detailed Explanation)
These are
some of the major aspects on which polarity of the molecules are used to
determine:
Electronegativity difference
Electronegativity
is the tendency of an atom to attract bond pairs of electrons. Different atoms
have different electronegativity values. Higher the electronegativity value, the more
closer atom can pull the bond pairs of electrons.
If there is an electronegativity difference between two atoms, partial positive and negative
charges induced on both ends means atom that has a lower EN value has positive
charge and higher EN value have a negative charge induced.
In the case
of AsF5, there are a total of five bond pairs in the form of As-F. Now,
Electronegativity
value of As = 2.16
Electronegativity
value of F = 3.98
Electronegativity
difference = 3.98 – 2.16 = 1.82
1.82 the electronegativity difference between As and F bonds which means As-F bonds are
polar according to the Pauli scale. But due to the symmetrical structure of
AsF5, the molecule will be nonpolar.
Lewis Structure of AsF5
Lewis structure
is a pictorial representation of a molecule where each atom’s valence electrons
are placed according to the octet rule. Lewis structure of a molecule helps in
the figure out the molecular geometry, bond formation, boiling & melting
point, etc.
In AsF5,
there are a total of 40 valence electrons present (35 from five fluorine atoms
and 5 from the arsenic atom). The complete Lewis dot structure is shown in the above
figure.
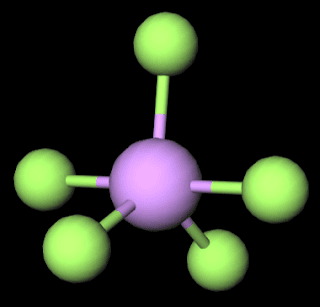
Molecular Geometry of AsF5
Arsenic
pentafluoride has a trigonal bipyramidal molecular geometry which is considered
as a symmetrical geometry. And this is the main reason why AsF5 is a nonpolar
molecule although it has five polar bonds in the form of As-F.
The induced
polarity on each As-F bond gets canceled by each other as the molecule is symmetrical.
And we know that dipole charge is a vector quantity so that the results of
each dipole charges becomes zero.
Dipole Moment of AsF5
Dipole moment
is defined as the product between induced charge (Q) and the distance between the
atoms (d).
Dipole
moment = Charge (Q) * distance between the atoms (d)
Here in AsF5,
the induced charge on the entire molecule is zero which makes the dipole moment
also zero. The dipole moment of the molecule is zero means it is a nonpolar
molecule.
Conclusion
AsF5
(Arsenic pentafluoride) is a nonpolar molecule because it has a symmetrical
trigonal pyramidal molecular geometry which makes the net dipole charge zero. However,
all five As-F bonds are polar but the net polarity of the molecule becomes zero
as polarity a vector quantity.
This is all
about the article “Is AsF5 Polar or Nonpolar?”. If you have any doubts, connect
with us (by subscribing through the above SUBSCRIBE bottom), you will get an instant response
on your email.


Post a Comment