Rubidium, a chemical element with a symbol Rb, is one of the highly reactive alkali metals of group 1 with atomic number 37 in the periodic table. Rubidium is not found in a free state in nature due to its high reactivity behavior so that it is abstracted from different compounds (mostly from salts).
As we know how much rubidium is being used in the world of chemistry, so we must have very good proper information about its electronic properties to survive in the world of chemistry and that’s why you are here to know what valence electrons and valency of rubidium are, aren’t you? But for this you have to know what these two terms are, so without wasting your time let's go for it,
Difference between valence electrons and valency
Valence electrons are the total number of electrons present in the outermost shell of an atom (i.e. in outermost orbital). The valence electrons for a neutral atom is always definite, it cannot be varied (more or less) in any condition for a particular atom and may or not be equal to its valency.
Valency is defined as the total number of electrons an atom can lose, gain, or share at the time of bond formation to get a stable electronic configuration i.e. to complete an octet. The valency of an atom can be variable in different compounds or in chemical reactions due to different bonding mechanisms.Rubidium (Rb) valence electrons
There are four simple steps to find out the valence electrons for rubidium atom which are:
Step 1: Find the Atomic Number
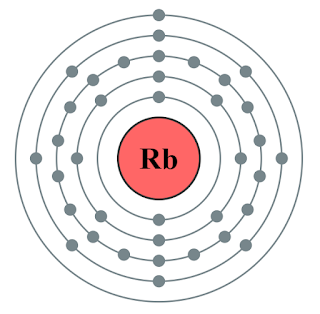
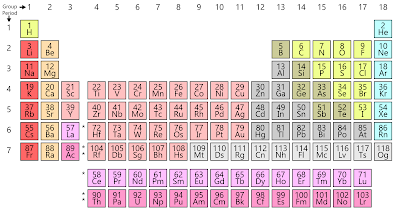
To find out the atomic number of rubidium, we can use the periodic table. With the help of the periodic table, we can easily see that the atomic number of rubidium is 37. As its atomic number is 37, it has a total of 37 protons, and for neutral rubidium, the number of protons is always equal to the number of electrons i.e. 37 electrons in the nucleus.
Step 2: Write Electron Configuration
Electron configuration is the arrangement of electrons on the orbitals. The rubidium atom has a total of 37 electrons, so we have to put 37 electrons in orbitals. The first two electrons will go in the 1s orbital as S orbital can hold a maximum of two electrons only. The next two will go in 2s orbital and the next six electrons will go in 2p orbital as P orbital can only hold a maximum of 6 electrons and so on...
Step 3: Determine Valence Shell
As we know, the valence shell of an atom can be found from the highest number of principle quantum numbers which is expressed in the term of n and in 1s2 2s2p6 3s2p6d10 4s2p6 5s1, the highest value of n is 5 so that the valence shell of Rb is 5s1.
Step 4: Find Valence Electrons
The total number of electrons present in the valence shell of an atom is called valence electrons, and there is only one electron present in the valence shell of rubidium (5s1). Thus, rubidium has only one valence electron.
Valency of Rubidium (Rb)
There are many different ways to find out the valency of an
atom which reflects the ability of an atom to bond with other atoms. Valence describes
how easily an atom or a free radical can combine with other chemical species. The
valency of an atom is determined based on the number of electrons lost, gained, or shared with another atom.
An atom is said to be stable when its outermost shells have
eight electrons (except H and He). If the total number of electrons in
outermost shells is between one to four, the atom has positive valency and if
electrons are between four to eight, the valency is calculated by subtracting
from eight and valency is negative. Atoms having four outermost electrons
possess both positive and negative valency and atom having eight outermost
electrons have zero valencies (i.e. noble gases).
Alkali metals like rubidium reached the stable (nearest inert gas configuration) by losing one outermost electron. So that the valency of rubidium (Rb) is 1.
We can also find the valency of rubidium with the help of a periodic table. As rubidium is an element of group 1 which indicated alkali metals group and valency of alkali metals are always 1.
Valence electrons and valency of Rb+
Rubidium-ion Rb+ means it has lost one electron and has only 36 electrons in the orbitals. The electron configuration of neutral Rb is 1s2 2s2p6 3s2p6d10 4s2p6 5s1 but in Rb+ it loses one electron, so it has a new electron configuration of 1s2 2s2p6 3s2p6d10 4s2p6 means Rb+ has (2+6=8) outermost electrons which makes it stable. Thus, rubidium ion (Rb+) has eight valence electrons.
Rb+ valency is not zero like noble gas as their outermost shell has eight electrons. when a rubidium atom loses one electron, Rb+ ion is produced and that’s what valency is. So that Rb+ valency is +1 not zero.
Chemical Properties
Rubidium (Rb) atom| Atomic number | 37 |
| Number of protons | 37 |
| Number of electrons | 37 |
| Electron configuration | 1s2 2s2p6 3s2p6d10 4s2p6 5s1 |
| Valence electrons | 1 |
| Valence/Valency | 1 |
| Number of electrons | 36 |
| Electronic configuration | 1s2 2s2p6 3s2p6d10 4s2p6 |
| Valence electrons | 8 |
| Valence/Valency | +1 |

Post a Comment