In this article, we will learn how to solve reaction box problems for choosing the best reagent and conditions. Basically, the solution to these types of problems are very simple due to huge similarities and repeated patterns but we have to be very careful because of the different conditions and structures.
How To Solve "Place The Best Reagent In Each Reaction Box" Problems
These are some of the major steps you have to follow while
solving reaction box problems:
Step 1: Analysing the reaction
This is an observational process where you have to analyze
the given reaction. You can easily figure out compounds are:
- Saturated or unsaturated
- Aliphatic or aromatic
Step 2: Count the number of carbons on both sides
Here you have to just
count the number of carbon present on both sides and find out whether carbon numbers are decreased or increased.
Step 3: Figure out the IUPAC name of given compounds
Try to write correct IUPAC names of both compounds which
will help you to know the behavior of the compounds as well as the type of
reaction applied.
Step 4: Choose the best reagent and conditions
Choosing the best reagent and conditions are most difficult
steps in such problems. But if you had done the above three steps correctly, no
need to be worry. Just focused on the given reagents and conditions, and figure
out:
- Oxidizing and reducing agents
- Acidic and basic reagents (if a molecule have more the one acidic proton and our need is to add some base to it then we should know which proton will be abstracted first and so on.)
Step 5: Know basic named reactions types
For any conversion in an organic compound, you must have some
knowledge about basic reaction types that are frequently used in the organic
chemistry. Here are some of the basic named reactions that are mostly used and
asked:
- Halogenation (Eg. Hoffmann Bromination)
- Oxidation and reduction
- Nitration
- Hydrolysis
- Carboxylation and Alkyl Cyanide formation (used if you want to increase carbon numbers)
- Hunsdiecker reaction and iodoform preparation (used if you want to reduce carbon numbers)
These are some additional famous reaction types methods:
- Markovnikov and Anti-Markovnikov methods
- Heinsberg’s method
- Ozonolysis
- Grignard’s method
- Functional group rearrangements
Now, based on the above five steps, we have some different
types examples for the problem “In Each Reaction Box, Place The Best Reagent And
Conditions From The List Below” which will definitely clear all your doubts and
you will be master in such problems.
Example 1: (Three boxes total)
Answer:
Step-by-step explanation:
1. On the left side, the given structure has two carbon with a triple bond, which means it is an alkyne named acetylene or ethyne. And on the right side, the structure has four carbon with a single bond and two bromine atoms are attached with the same carbon atom which IUPAC name is 2,2-dibromo butane.
2. Here the number of carbons is increased and the triple bond is also changed into single bonds. So first, we have to use sodium amide (NaNH2) which gives acetylide ion which an excellent nucleophile. This is because it can easily react with alkyl halides to form a new carbon bond.
3. Our final product has four carbon means we need to add two more carbon so CH3-CH2-Br is the only choice we have. Here we will get 1-butyne but our final product doesn’t have a triple bond and have two bromine attached on the same atom.
4. Now we wanted to add two bromine groups and reduce triple bonds into single bonds. For that, we should add two equivalents of HBr (i.e. Br2 2-equiv.). in this process, the first one bromine reduces triple bond to double bond and the second bromine reduces double bond to a single bond. And here we will get our final product successfully.
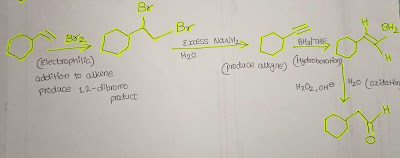
Example 2: (Four boxes total)
Answer:
Step-by-step explanation:
1. In this given conversion reaction, an aromatic compound
that is attached with an alkene ended with an additional -OH functional group at
the end. These are our general analysis.
2. So, we have to use an electrophile which is Br2 to get
1,2- dibromo product.
3. When we use excess NaNH2 and then H2O to 1,2-dibromo
products, it will produce alkyne.
4. Now its time for the hydroboration means when we add BH3/THF
to an alkyne, the triple bond is changed to a double bond.
5. To add an -OH functional group, we have to do oxidation
in the presence of H2O2, NaOH, and H2O which will give us the final structure we
needed.



Post a Comment