Xenon dichloride is the chemical name of XeCl2 and this is the only stable chlorine xenon compound.
This is prepared by reacting xenon and fluorine gas together
in the presence of microwave discharge.
Xe + Cl2 → XeCl2
There are many doubts regarding ‘is xenon dichloride is a true
compound? or it is just a van der Waals force of attraction between xenon and
chlorine atom? because in this molecule chlorine atom is connected by a
secondary bond.’
Here in this article, we are going to know whether XeCl2 is
a polar or nonpolar molecule with a detailed explanation. But before entering into XeCl2
polarity, let’s have some ideas about what polar and nonpolar molecule are:
Polar Molecules
Polar molecules are those molecules that have a net dipole
moment and this is possible only if there is an electronegativity difference
between the associated atoms as well as asymmetrical geometry so that the induced
dipole charges do not cancel each other.
There are some
certain conditions for the molecule to be polar i.e. have asymmetrical
structure, presence of lone pair of electrons or electronegativity difference
between central atom to bonded atoms.
Polar molecules have polar covalent bonds or ionic bonds.
They are soluble in water (called hydrophilic) and other polar solvents like acetic
acid (CH3-CO-OH), methanol (CH3-OH), etc.
Nonpolar Molecules
Nonpolar molecules are considered as a pure covalent bond
because it forms by equal sharing of electrons between the atoms in a compound
and this is what a covalent bond is known for.
One more case for the nonpolar molecule if the molecules
have symmetrical structure so that the induced partial charges on the
individual atoms nullify each other and the net dipole moment becomes zero.
As these are a pure covalent bond so they are insoluble in
water (called hydrophobic) and other polar solvents but are soluble in nonpolar
solvents like carbon tetrachloride (CCl4), acetone (C3H6O), benzene (C6H6), etc,
and these also are called lipophilic as they are soluble in fats, oils and
greases.
Now, is XeCl2 polar or nonpolar? XeCl2 is a nonpolar molecule despite two Xe-Cl bonds are polar. The XeCl2 has a linear molecular geometry and Xe-Cl bonds are symmetrical (180°) to each other, as a result, the net dipole moment becomes zero.
Why is XeCl2 a nonpolar molecule? [Detailed Explanation]
As we have already known there are certain conditions for a
molecule to be polar or nonpolar in the above definitions of the polar molecule and
nonpolar molecule. The same things apply here which makes XeCl2 a molecule.
In XeCl2, there are two chlorine atoms are bonded with a central xenon atom. Also, there are three lone pair of nonbonding electrons
are localized on the central Xe atom arranged symmetrically.
The Xe-Cl bonds are polar as the electronegativity difference
between xenon (2.6) and chlorine (3.16) is 0.56. But we know molecular polarity
is a vector quantity which means the net polarity is the vector sum of
individual induced bond dipoles.
The molecule is symmetrical so that all the Xe-Cl bond
induced dipoles are opposing each other, as a result, net dipole moment on the
molecule becomes zero.
But as shown in the above diagram, there are still three pairs
of non-bonding electrons are present on central Xe atom which is aligned in
equatorial position so that their effects are also nullified. And there is not
any dipole charge presence on the molecule which makes XeCl2 a nonpolar
molecule.
Also Read: Is XeF2 Polar or Nonpolar?
XeCl2 Polar or Nonpolar (Based on polarity determining factors)
These are some of the major factors which are used to determine the polarity of the compounds:
Electronegativity difference
Electronegativity is a kind of force exerted by atoms on
their bonding partners at the time of bond formation. All the atoms have their
own electronegativity value which varies from atom to atom. Some have a higher EN value
and some have lower EN values.
The atoms having higher electronegativity value attract the
electrons pair from the bonding partners. It means the higher the electronegativity
difference between atoms, the more uneven sharing of electrons takes place. In the
periodic table, the fluorine (F) atom has the highest electronegativity value 4.0, and
francium (Fr) has the lowest 0.7.
In the XeCl2 compound, the electronegativity difference between
fluorine (3.16) and Xe (2.6) is 3.16-2.6= 0.56 which shows the Xe-F bond is polar
according to the Pauli scale.
Molecular geometry
The central atom of XeCl2 (Xe) has a total of 10 electrons
localized in the form of 4 as Xe-Cl bonding and 6 as non-bonding pairs.
And due to the symmetrical structure, the polarity of the Xe-Cl bond cancel each other and no dipole charge induced on the molecule.
Dipole moment
The dipole moment is defined as the products of induced charge
and distance of separation between the atoms. It is given as,
Dipole moment = Charge (Q) * distance of separation (d)
Its unit is Debye and denoted by ‘D’. 1D = 3.33564*10-30
C.m, where C is Coulomb and m is meter.
The dipole moment is the major aspect for any compound to be
polar or nonpolar. All the polar molecules have a net dipole moment and all
nonpolar have zero dipole moment.
XeCl2 is a nonpolar molecule so that its dipole moment is 0D (Zero).
Properties of XeCl2
- Its molar mass is 202.199 g/mol and has a total of 22 valence electrons present.
- It has a linear geometry where the bond angle between Xe-Cl bond is 180°.
- XeCl2 hybridization is sp3d types and Xe oxidation state is +2.
Uses of XeCl2
- Xenon dichloride is used in excimer lager with xenon monochloride (Xe2Cl).
- It is used as fuel in ion engines.
This is all about the article “Is XeCl2 Polar or Nonpolar?”.
If you have still any doubts or queries, feel free to ask us but for that, you
have to make sure you have already subscribed to Science Coverage (Subscribe
bottom at the top of the page). Get premium support! Don’t forget to check your
Gmail account after successfully subscribed. Something is there for you, hurry
up!
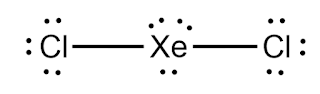


Post a Comment