Methane colorless, odourless, gas that occurs abundantly in nature and as a product of certain human activities. It is the member of alkane series and also termed as greenhouse gas. Methane is a saturated hydrocarbon.
Its next name is marsh gas because it is found in swamps or marshy lands where it is produced by bacterial decomposition of complex vegetables and animal matters. it is the simplest and first member of the alkane series.
It also occurs in coal mines, where it forms an explosive mixture with air and, therefore miners called it as fire damp. Natural gas obtained from petroleum wells contains 82.98% methane which is used as an domestic fuel.
Methane also occurs in gobar gas and bio-gas, which are formed by the decomposition of dungs of animals, excreta of humans beings and plant wastes.
It’s chemical formula is CH4.
Some properties of CH4 are :- non-polar compounds and
they are insoluble in water but soluble in organic solvents such as alcohol,
ether, acetone etc.
Liquid hydrocarbons are lighter than water.
It has melting point -182.5°c and -161.5°c boiling
point.
It is principal component of natural gas and is primarily used as fuel to make light and heat
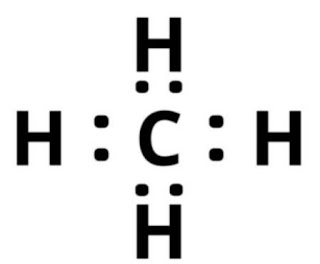
CH4 (methane) Lewis structure
Lewis structure is a very simplified representation of valence electrons in a chemical species like an atom, ion, or molecule. It indicates how electron are situated around the atoms either as lone pairs o bond pairs.
In lewis structure dot do represent lone pairs of electrons and
line represents bond pairs of electrons.
Lewis electronic/dot structure of
molecule or ions are written based under octet rule. For this purpose the
following are the requirements.
·
Valence electron of some of the common atoms of
‘s’ and ‘p’ block
·
Bond between metal and non-metal is
electrovalent and between two metal is covalent.
· Total number of electrons are required for writing the structures are obtained by adding valence electrons of the combining atoms.
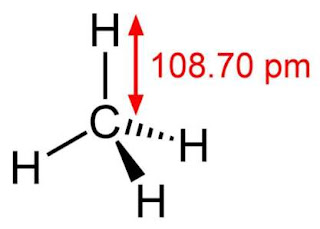
In methane, the central atom carbon has four bonded electron pairs. It forms four equivalent covalent bonds with four hydrogen atoms. The four bonds are directed towards the four corners of a rectangular tetrahedron.
Since all the four bond pairs are equivalent, the molecule according to VSEPR theory has regular geometry of tetrahedron with a bond angle H—C – H as 109.5°
Before drawing lewis structure, we must have better knowledge about valence electrons, octet rule, and formal charge for understanding better.
WHAT IS VALENCE ELECTRON OR VALENCE SHELL :-
Electrons present in the outermost shell of an atom are valence electrons and the shell is called valence shell. Valence shell electrons decide the combining capacity of an element and those electrons only take part in the formation of chemical bond. Inner electrons have nothing to do with the bond formation.
Inner
electrons are called core elements. The nucleus and inner electrons is
positively charged ‘kernal’ according to lewis.
According to VSEPR valence electron of methane is 8. i.e. 4 valence electrons in the central atom and 4 HYDROGEN each contribute 1 makes 4 so on adding both we get 8.
WHAT IS OCTET RULE ?
Elements with eight electrons in their valence shell are said to be most stable form of electronic configuration and such element do not take part in bond formation. Inert gas elements (except He where it is duplet ) possess such stable electronic configuration in their atomic state. So they show vary little or no tendency to react with other elements.
Rest of the elements do not have octet state and they tend to acquire eight electrons in their valence shells so as to give the similar stable electronic configuration of the nearest inert elements. This is octet theory or octet state and it is the main cause of chemical combination.
For carbon atom in methane, octet state isn’t satisfied as carbon has 4 electrons in its outermost valence shell. In CH4 carbon has one covalent bond with each of the four hydrogen which share the electron with carbon atom so in total carbon has total 8 electrons and for hydrogen in CH4, duplet rule is satisfied as hydrogen has only one electron in its outermost valence shell.
Each of 4 hydrogen in CH4 has one covalent bond with the central carbon atom so each of them share one electron with carbon. Thus, their valence shell contains 2 electrons and satisfy duplet rule.
WHAT IS FORMAL CHARGE ?
The formal charge present on the ion is the charge on
that ion as a whole and is not related to the individual atoms. However, it is
also possible to assign a formal charge to an atom present in the ion or
in the molecule if it is required.
Following is the simple rule of calculation can be employed for formal charges.
FORMAL CHARGE = [ total number of valence electron(VE)] – [ total number of non-bonding electrons(lone pairs of electrons)(NBE)] – ½[total number of bonding electrons shared ( BE )]
STEPS FOR DRAWING LEWIS STRUCTURE OF CH4
Step 1 : count the total number of valence electrons
present on each atom of CH4 molecule.
As we know, carbon is of IVA group element and contains 4
elements in its last shell and hydrogen a group of IA elements and has only one
electron in its last shell so now we have known total valence electron in their
respective valence shell of hydrogen and carbon atoms.
·
Valence electron by hydrogen = 4*1 = 4
·
Valence electron by carbon = 1*4 = 4
·
So total valence electrons = 8
Step 2 : determining the total electrons pairs existing
as lone pairs and bonds
Total valence electron pairs = ϭ bond + bonds
+ lone pairs at valence shells
Total electron pair can be determined by dividing the number total valence electrons by two.
Step 3 :
center atom selection
As hydrogen atom cant be a center atom, carbon becomes center atoms because hydrogen has its maximum valence one
Step 4 : marking lone pair on atom
After finding center atom and sketch of CH4 molecule, we can
start to mark lone pairs on atoms. There must be total of four electron pairs
as lone pairs and bonds
· There are already present of C – H bonds in the above sketch. So, there are no more electrons pairs to mark on atoms
Step 5 : marking charges on atoms if there are any charges
As shown in the structure, we can determine that there are
no any charges on carbon and hydrogen atoms
Step 6 : calculate formal charge
[formal charge]H = 1 – (1/2)*2 – 0 = 0 this applies to each hydrogen and these hydrogens all are 0 so molecule is neutral.
checking the stability and minimizing charges on
atoms by converting lone pairs to bonds
In the above drawn structure there are no any presence of charges on both atoms i.e. hydrogen and carbon atoms . so, we have obtained the lewis structure of CH4
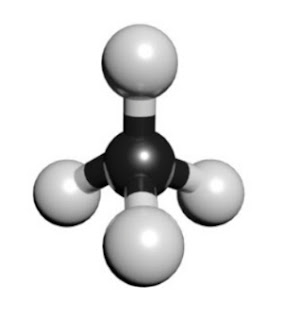
CH4 MOLECULAR GEOMETRY AND SHAPE
Lewis structure of methane shows that the central atom C has four bonding electron pairs. These electron pairs repel each other and are thus directed to the four corners of a regular tetrahedron. A regular tetrahedron is a solid figure with four faces which are equilateral triangles. All bond angles are 109.5° .
It satisfy VSEPR theory because as per VSEPR theory molecular shape only consider bond pairs or atoms while electron geometry considers bonded atoms as well lone pairs present on the central atom. And follow AX4 generic formula.
CH4 Hybridization
To explain fully the tendency of these atoms to form bonds and the shape or geometry of their molecules, a new concept was introduced called hybridization. Hybridization’s concept tells, we may mix any orbital. The mixing orbital generally belong to the same energy. The main characteristic of hybrid orbital is that they are energetically and directionally identical; however different from the original atomic orbital.
In methane, carbon atom is sp³ hybridized giving four sp³hybrid orbital of equivalent energy. These orbital are directed in space to the four corners of a regular tetrahedron. The linear combination of four. So, the type of hybridization involved in CH4 is sp³.
CH4 polar or non-polar ?
For this we must have to know about electronegativity,
molecular geometry and dipole moment
Electronegativity : the tendency of an atom to attract
electrons to itself is called electronegativity of the atom. Electronegativity
is an inherently fundamental property of an atom and is fundamentally different
from electron affinity. Since, electron affinity represents the tendency of an isolated
gaseous atom to attract the electron while electronegativity represents the
tendency of a bonded atoms to attract the shared electron pair.
Molecular geometry : molecular geometry , also known as
dimensional structure or arrangement of atoms in a molecule. Understanding
molecular structure of a compound help to determine polarity, reactivity.
Dipole moment : dipole moment comes when there will be separation of charge. They occurs between two ions in an ionic bond or between atoms in a covalent bond; dipole moment arise from difference in electronegativity and dipole moment is a measure of the polarity of the molecule.
So, methane( CH4 ) is an non-polar as it has a symmetric tetrahedral geometrical shape with four identical C—H bonds.


Post a Comment