Xenon difluoride is powerful fluorinating agent with the chemical formula XeF2 and one of the most stable compounds. It is discovered in 1962 Like most covalent inorganic fluorides, it is moisture-sensitive.
It goes on decomposition(decomposes) on contact with water vapor but is otherwise stable in storage. It is a dense, colorless and crystalline solid. It has nauseating odour and low vapour pressure.
The oxidation state of xenon in Xenon Difluoride is +2. Xenon tetrafluoride has a negligible vapour pressure at -78° and about 3 mm-Hg at room temperature. It is an hazardous chemical, reacting with water moisture to form hydrofluoric acid.
·
It
has molecular mass/molar mass of 169.29g/mole
·
It
has boiling point of 155°c
·
It
has melting point of 128.6°c
· It has density of 4.32g/cm̤³.
Lewis structure of XeF2(XENON DIFLUORIDE)
Lewis structure is a very simplified representation of valence electrons in a chemical species like an atom, ion, or molecule. It indicates how electron are situated around the atoms either as lone pairs o bond pairs. In lewis structure dot do represent lone pairs of electrons and line represents bond pairs of electrons.
Lewis electronic/dot structure of
molecule or ions are written based under octet rule. For this purpose the
following are the requirements.
·
Valence electron of some of the common atoms of
‘s’ and ‘p’ block
·
Bond between metal and non-metal is
electrovalent and between two metal is covalent.
·
Total number of electrons are required for
writing the structures are obtained by adding valence electrons of the
combining atoms.
·
For anions, each negative charge indicate
addition of one electron and positive charge means substractions of s electron
to valence electrons.
·
Total number of covalent bonds including
coordinate bonds formed in a given molecular formula is obtained as
Number of bonds = total number of
valence electrons after saturation – before saturation / 2
Keeping these information in mind, lewis structure of a
molecule is written by showing valence electrons of all atoms except cations
with dots and cross notation.
· Least electronegative atom occupies central position in molecule/ion. For example = in NF3 , N is central atom and in Co3--, C is central atom.
WHAT IS VALENCE ELECTRON OR VALENCE SHELL
Electrons present in the outermost shell of an atom are valence electrons and the shell is called valence shell. Valence shell electrons decide the combining capacity of an element and those electrons only take part in the formation of chemical bond.
Inner electrons have nothing to do with the bond formation. Inner electrons are called core elements. The nucleus and inner electrons is positively charged ‘kernal’ according to lewis.
WHAT IS OCTET RULE ?
Elements with eight electrons in their valence shell are said to be most stable form of electronic configuration and such element do not take part in bond formation.
Inert gas elements (except He where it is duplet ) possess such stable electronic configuration in their atomic state. So they show vary little or no tendency to react with other elements.
Rest of the elements do not have octet state and they tend to acquire eight electrons in their valence shells so as to give the similar stable electronic configuration of the nearest inert elements. This is octet theory or octet state and it is the main cause of chemical combination.
WHAT IS FORMAL CHARGE ?
The formal charge present on the ion is the charge on
that ion as a whole and is not related to the individual atoms. However, it is
also possible to assign a formal charge to an atom present in the ion or
in the molecule if it is required.
Following is the simple rule of calculation can be employed for formal charges.
FORMAL CHARGE = [ total number of valence electron(VE)] –
[ total number of non-bonding electrons(lone pairs of electrons)(NBE)] –
½[total number of bonding electrons shared ( BE )]
Formal charge on Xef2 = 8-6-2=0 and when looking back to lewis structure and formal charge we can be able to get known that formal charge becomes 0 and makes the best lewis structure best
STEPS FOR DRAWING LEWIS STRUCTURE OF XeF2
STEP 1 : Determine the total number of valence electrons
As we know Xenon lies in group 18 so it has valence electron
8
Fluorine has 7 valence electron although we have 2 fluorine
so, 7*2 = 14
So total valence electron given by xenon and fluorine = 8 + 14 = 22
STEP 2 : Draw a sketch/outline by joining the atoms by single bonds. Xenon will
be the central atom
as fluorine cannot be a central atom
f – Xe – f
STEP 3 : distributing remaining electrons.
We have placed three lone pairs of electrons around each Fluorine atom, accounting for the 12 electrons and giving each Fluorine atom 8 electrons. Thus, six electrons (three lone pairs) will remains. These lone pairs now should be placed on the Xe(Xenon) atom.
And this was acceptable because Xe atoms have empty valence shell d-orbitals and can accommodate more than eight electrons. The Lewis structure of XeF2 shows two bonding pairs and three lone pairs of electrons around Xe atom
Since XeF2 violates the octet rule, there is no need to go on more steps.
H2 MOLECULAR GEOMETRY AND SHAPE
According to VSEPR the molecular geometry and shape of XeF2 is linear as it gain such shape as lone pairs present around central atom tries to take equatorial position and the bond angle was made 180°
XeF2 Hybridization
To explain fully the tendency of
these atoms to form bonds and the shape or geometry of their molecules, a new
concept was introduced called hybridization. Hybridization’s concept tells, we
may mix any orbital. The mixing orbital generally belong to the same energy.
The main characteristic of hybrid orbital is that they are energetically and
directionally identical; however different from the original atomic orbital.
The arrangement of the electrons of Xenon is changed into s2p5d11 with two unpaired electrons so, the hybridization of central atom Xe is sp3d.so, hybridization of XeF2 is sp3d.
XeF2 polar or non-polar ?
For this we must have to know about electronegativity,
molecular geometry and dipole moment
Electronegativity : the tendency of an atom to attract electrons to itself is called electronegativity of the atom. Electronegativity is an inherently fundamental property of an atom and is fundamentally different from electron affinity.
Since, electron affinity represents the tendency of an isolated
gaseous atom to attract the electron while electronegativity represents the
tendency of a bonded atoms to attract the shared electron pair.
Molecular geometry : molecular geometry , also known as
dimensional structure or arrangement of atoms in a molecule. Understanding
molecular structure of a compound help to determine polarity, reactivity. The
molecular geometry of XeF2 is Linear as bond angle is 180°
Dipole moment : dipole moment comes when there will be separation of charge. They occurs between two ions in an ionic bond or between atoms in a covalent bond; dipole moment arise from difference in electronegativity and dipole moment is a measure of the polarity of the molecule.
So from above sketch, XeF2 is non-polar because it has linear shaped geometry having fluorine atoms symmetrically on both side of the xenon atom.
But Xe-F bond is polar as the electronegativity of Xe and F is unlike but the polarity of both Xe-F bonds gets cancelled by each other resulting in a non-polar XeF2 molecule.

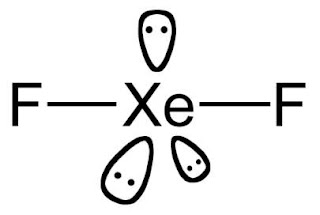



Post a Comment